Congratulations to members of the McCauley and Darbar labs for publishing this important work in Circulation Arrhythmia and Electrophysiology linking metabolism to ion channel function in atrial fibrillation. These results will help inform which anti-arrhythmic drugs may be of best use in patients with metabolic syndrome. See the abstract, graphic, and link below.
Ion Channel and Structural Remodeling in Obesity-Mediated Atrial Fibrillation
Background:
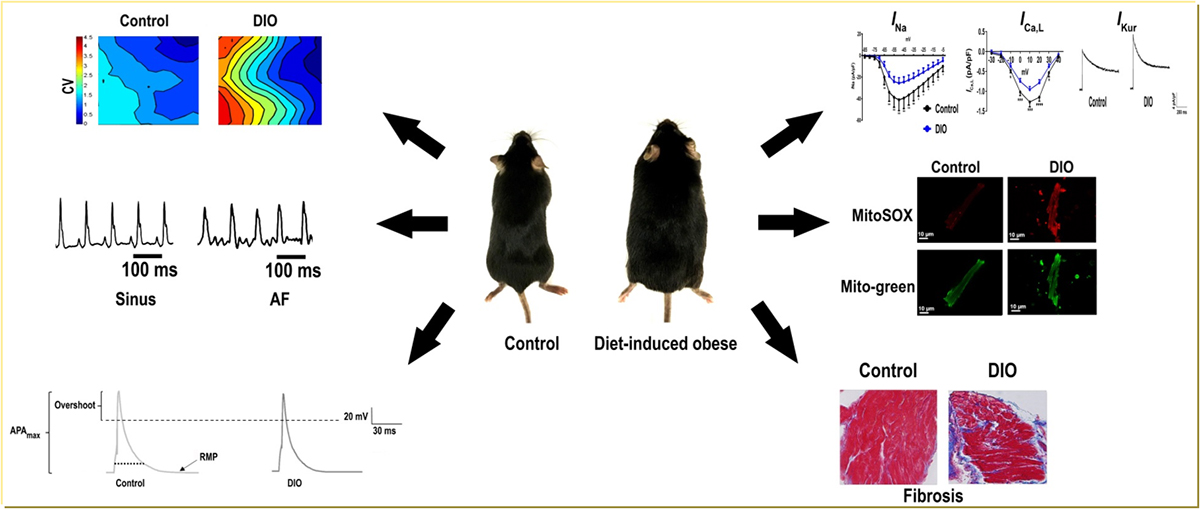
Epidemiological studies have established obesity as an independent risk factor for atrial fibrillation (AF), but the underlying pathophysiological mechanisms remain unclear. Reduced cardiac sodium channel expression is a known causal mechanism in AF. We hypothesized that obesity decreases Nav1.5 expression via enhanced oxidative stress, thus reducing INa, and enhancing susceptibility to AF.
Methods:
To elucidate the underlying electrophysiological mechanisms a diet-induced obese mouse model was used. Weight, blood pressure, glucose, F2-isoprostanes, NOX2 (NADPH oxidase 2), and PKC (protein kinase C) were measured in obese mice and compared with lean controls. Invasive electrophysiological, immunohistochemistry, Western blotting, and patch clamping of membrane potentials was performed to evaluate the molecular and electrophysiological phenotype of atrial myocytes.
Results:
Pacing-induced AF in 100% of diet-induced obese mice versus 25% in controls (P<0.01) with increased AF burden. Cardiac sodium channel expression, INa and atrial action potential duration were reduced and potassium channel expression (Kv1.5) and current (IKur) and F2-isoprostanes, NOX2, and PKC-α/δ expression and atrial fibrosis were significantly increased in diet-induced obese mice as compared with controls. A mitochondrial antioxidant reduced AF burden, restored INa, ICa,L, IKur, action potential duration, and reversed atrial fibrosis in diet-induced obese mice as compared with controls.
Conclusions:
Inducible AF in obese mice is mediated, in part, by a combined effect of sodium, potassium, and calcium channel remodeling and atrial fibrosis. Mitochondrial antioxidant therapy abrogated the ion channel and structural remodeling and reversed the obesity-induced AF burden. Our findings have important implications for the management of obesity-mediated AF in patients.

Recent Comments